The quality system set up using the Good Distribution Practice, based on the quality assurance model system for supply agencies and the ISO 9001 standard helps to control all the activities with an impact on quality with the aim of permanent compliance of products and services, customer satisfaction and compliance with regulations.
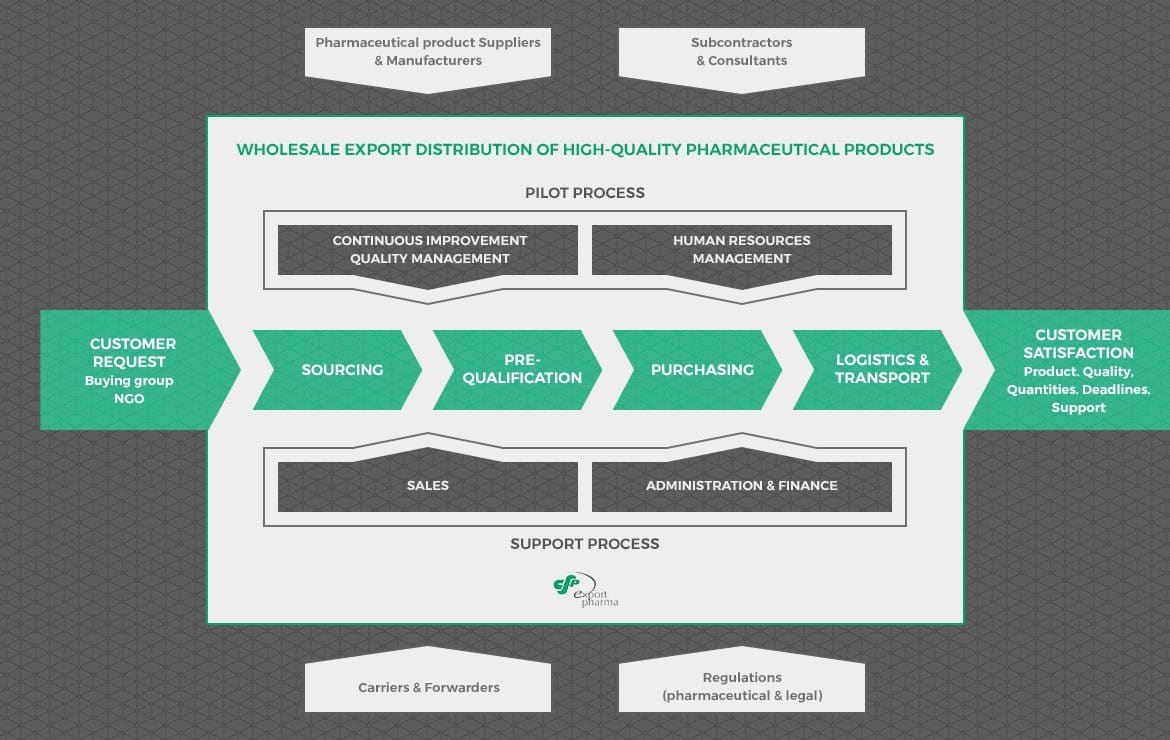
In order to meet these aims, CSEP is structured into 4 production processes (sourcing, pre-qualification, purchasing and logistics/transport) and steered by quality management, continuous improvement and human resources. The essential support processes for the company’s work are the sales, administrative and financial processes.
Each year, the management carries out a formal assessment of the state of the quality system and how well it measures up to the quality policy and its aims.
The general indicators connected to the quality system and the specific indicators for each of the processes are assessed with the aim of continuous improvement.
Supplier assessment
After the sourcing stage, the pharmaceutical product suppliers are all assessed by the quality department using different methods depending on the risk:
– Good Manufacturing Practice certificates
– Document analysis
– Manufacturing site audits and assessment of corrective and preventive actions plans
The final decision on supplier pre-qualification is made by a committee, which guarantees that decisions are made independently. Performance of the pre-qualified suppliers is assessed at a regular, defined frequency.
Product evaluation.
Documentation regarding pharmaceutical products is requested from the different pre-qualified suppliers. The quality department assesses the analysis certificates (primary materials and finished products), the specifications (primary materials and finished products), the stability studies, the proof of effectiveness (according to dosage form) and the instructions and labelling according to the predefined operating modes.
Document assessment results are included in a pharmaceutical product pre-qualification database. This database, developed by CSEP, means that a score can be given to each pharmaceutical product included in this tool. The score takes into account all the quality criteria being assessed, weighted according to risk.
A quality control policy is implemented depending on the score given to each product and the manufacturers’ level of risk.
